INFORMATION IN ORDER TO MAKE AN INFORMED DECISION ON THE TYPE OF CLEANING MATERIAL THAT NEEDS TO BE USEDHOW DIRTY IS DIRTY AND HOW CLEAN CAN YOU CLEAN IT?To understand how any cleaning product works we must understand what dirt is or rather what it is comprised of. Dirt is actually a layer of fine films made up of fats, oils, and grease (FOG), bacteria, fungi, dust mites, non-organic material and other organic micro-organisms. These layers or films are bonded to each other and to the surface by amino and fatty acids.
FOG is a combination of plant and animal fats known as lipids as well as mineral oil products which are all organic in origin. The method used in most cleaning solutions is to emulsify FOG, which is to put it into an emulsion or solution such that it can be relocated elsewhere through rinsing. Most cleaners emulsify some of these dirt films but may not break down the lower levels held together by amino and fatty acids. Usually the top layers of the fills are removed but some of the lower levels are left to collect bacteria. As a result, re-soiling can occur much faster. The primary function of cleaning is to reduce dirt, dust, bacteria and moulds from surfaces. Unfortunately modern societies have developed a cleanliness and ‘germ hating’ obsession which has lead to the development of ever more powerful cleaning agents. Millions of kilograms of general purpose cleaners are consumed worldwide each year. HEALTH & ENVIRONMENTAL HAZARDS OF SYNTHETIC CLEANING PRODUCTSSynthetic Cleaning Products use chemicals that are not just toxic if we accidentally eat or drink them. The volatile organic chemicals (VOC’s) contained in the products enter our indoor air and lungs as we use them, and many other ingredients are easily absorbed by the skin. They also indirectly affect our long-term health when they pollute our water ways.
Increasingly these chemical compounds are being associated with allergies and other common diseases that are growing in society. The increased use of chemical cleaning products has placed a significant burden on the environment in terms of wastewater loading and subsequent treatment, the emission of volatile organic compounds (VOCs), resource consumption and disposal of packaging materials. WHAT IS BIOAUGMENTATION?Nature employs bacteria as its cleaning staff to rid the environment of wastes. The bacteria consume these wastes as a food source and so purify the environment. Unfortunately, the modern world places a larger load of pollution and waste on the environment than it can cope with.
Bioaugmentation is the addition of bacteria which have been specially chosen for their ability to biodegrade these wastes (e.g. Sewerage, fats, and mineral oils). |
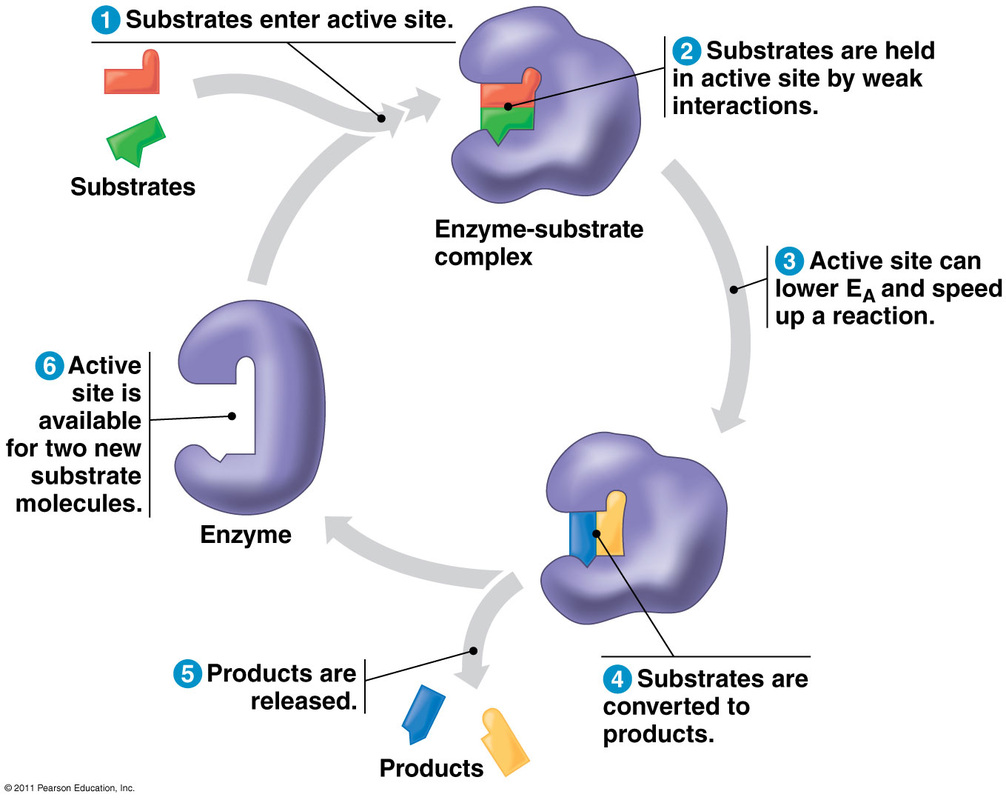
How Enzymes workEnzymes speed up reactions by lowering their activation energy, which is the energy required to jumpstart a chemical reaction. This enables the reactant molecules to reach the transition state quicker by absorbing enough energy to break their bonds and become unstable.
Another name for the reactant molecules is substrates. Substrates are what the enzyme acts on, and they bind to the enzyme to form enzyme-substrate complexes. There is a restricted region on an enzyme molecule to which a substrate will bind and where catalysis occurs. This region, usually an indention or pocket, is called the active site. The active site is formed by some of the enzyme protein's amino acids, and has a particular configuration which attributes to an enzyme's specificity. An enzyme must have a compatible fit between the shape of its active site and the shape of its substrate in order to carry out the chemical reaction. As temperature increases, the rate of an enzyme-catalysed reaction increases as well. However, once the enzyme exceeds its optimal temperature, denaturing takes place, which means their protein structure breaks down and the enzyme is deactivated. Just as an enzyme has an optimal temperature, it also has an optimal pH at which it is most active. There are a few exceptions, but pH values that optimize a majority of enzymatic activities fall in the range of pH 6-8, which is around neutral on the pH scale. A pH lower or higher than an enzyme's optimal pH results in denaturation of the protein. As long as the reaction is not limited by substrate availability, increasing the amount of enzymes will sufficiently increase the rate at which substrates are converted to products. Similarly, adding substrates to the reaction when the enzyme concentration is kept constant also boosts the rate at which the enzyme works. However, there is a maximum to be reached. At the maximum, all enzymes become occupied by a substrate; therefore, increasing substrate concentration beyond maximum will no longer increase reaction rate. For example, the substrates of the enzyme ecatecholase are oxygen and catechol, and the products formed by the reaction of these two reactants are benzoquinone and water. During the reaction, catechol is oxidized and oxygen is reduced. Benzoquinone molecules link together to form chains that create brown pigments, which is apparent when peeled fruits and vegetables turn brown when exposed to air. Email us to buy your AMA product |